The evaluation of the radiotoxicity of Gallium-68 Labelled Pharmacological compounds

Abstract
In vitro toxicity studies are important in pharmacological development studies, being performed before the testing of all newly synthesized drugs on animals, in order to provide relevant information regarding the effects such compounds can have on the organism. Thus, the toxicological tests are relevant in the development of certain radiopharmaceuticals, aimed for imaging and/or therapy.
Radiopharmaceuticals labelled with gallium-68 (68Ga), a positron emitter, developed for positron emission tomography, need to be able to generate a high-resolution image while having an as small as possible detrimental effect on the body, as part of the safety assessment of radiopharmaceutical preparation for human use.
The aim of this study is to compare the radiotoxic effects of a gallium-68 labelled somatostatin analogue peptide, Nal3-octreotide (NOC) on two tumour cell lines, U87MG (glioblastoma) and AR42J (pancreatic adenocarcinoma), both presenting an overexpression of somatostatin receptors.
The radiopharmaceutical investigated was 68Ga-NODAGA-NOC. The compound was synthesized
using a 68Ge/68Ga generator to obtain the gallium radioisotope, followed by the radiolabelling of NODAGA-NOC using an automated synthesis module. The radiotoxicity of 68Ga-NODAGA-NOC was assessed by testing the peptide affinity and retention on cell cultures using peptide-receptor interaction system, LigandTracer Yellow, followed by MTT testing of the cells viability.
Our analysis indicates a lesser level of peptide binding on the AR42J cells, but a higher level of retention compared with the U87MG cells. The cell viability test indicates a higher impact on the glioblastoma cells compared with the pancreatic adenocarcinoma.
68Ga-NODAGA-NOC is a peptide used for targeting of tumors with an overexpression of somatostatin receptor for medical applications. The in vitro testing paves the path for the development and improvement of radiolabeled compounds in medicine.
Table of Contents:
1. Introduction
2. Methodology
3. Results
4. Conclusions
1. Introduction
Used in nuclear medicine practice, radiopharmaceuticals are special class of medicines, which contain in their structure an unstable isotope. Such compounds, thanks to their radioactive decay properties, can be used in imaging techniques, for diagnosis or monitoring the disease progression, or for therapeutic purposes. Depending on the nature of the radioisotopes and the possibilities to integrate it in the molecular structure of a pharmacological compound, a number of different radiopharmaceuticals were studied and developed over time [1, 2].
The drug development process involves the selection of compounds showing active biological properties, such as preferential localization at the tumour site and/or high affinity to promoters of a certain pathological process, which would ensure the transport of the radioisotope to specific targets (organs, tissues, receptors). Particular interest is paid to early markers of a malfunction of an organ or any change in the physiological processes. A bi-functional chelating agent is needed to chemically link the radio-metal to the selected biomolecule, without interfering with the transport properties. The biological active component ensures the transport to the specific interaction site, while the chelator ensures the radionuclides are delivered to target. The overexpression of certain peptide receptors and molecules on the surface of cancerous cells have indicated the possibility of using radiolabelled peptides for diagnosis and treatment of oncologic disorders, leading to the development of molecular imaging and peptide receptor radionuclide therapy [3, 4].
Positron emission tomography (PET) is an imaging technique that involves the use of positron emitting radioisotopes resulting in tri-dimensional, functional images, mostly fused with morphological mapping such as CT or MRI. 18F is a widely used radionuclide for PET, but given the need for an on-site particle accelerator in order to obtain it and short half-life, its use can be limited [5, 6]. The studies of the 68Ga based radiopharmaceuticals is of great research interest, given the possibility to obtain the isotope using a more affordable and convenient method, the 68Ge/68Ga generators. 68Ga is a positron emitter with 68 min half-life; the annihilation gamma emission allows for high resolution images while the metal ion chemistry is straightforward.
2. Methodology
Radioligand synthesis
In this study a Ga-68 radiolabelled NODAGA-1-Nal-3-Octreotide conjugated somatostatin analogue peptide, supplied by PiChem Austria, was used (68Ga-NODAGA-NOC). The radioisotope is linked to the peptide through a metal ion chelator, which acts as a situs for the gallium isotope to bond in the structure of the peptide as shown in Figure 1. DOTA-NOC is a largely used somatostatin analogue in neuroendocrine tumours investigations and therapy, especially as carrier for the beta emitter 177Lu. In NODAGA-NOC, the DOTA is replaced by NODAGA, a chelator derivative from NOTA, which is the ‘gold standard’ for Ga3+ chelation because it forms more stable complexes than DOTA [7, 8].
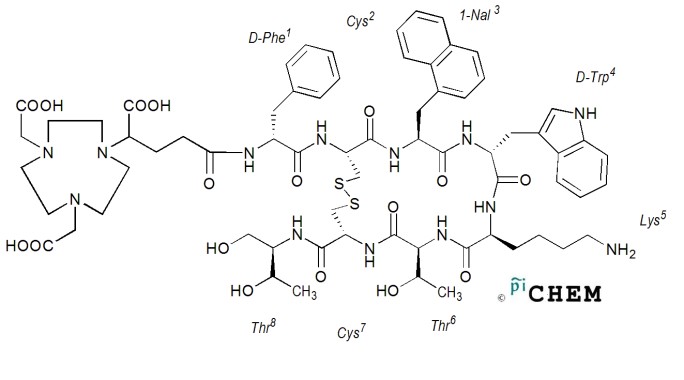
The 68Ga radioisotope was obtained from a 68Ge/68Ga generator as chloride, by elution with 5ml HCl 0.05 M. The solution was purified using the fractioned elution method [1, 9], the recovered 4, 5 and 6 fractions were used for the radiolabelling process. Binding the 68Ga to the peptide-chelator conjugate was performed using an automatic synthesis module (GaLigand 2, Comecer-Veenstra, The Netherlands). 22.58 nmols/50 μl of NODAGA-NOC were used for radiolabelling, at 95 °C, pH 3.8-
4.5, for 5 minutes. The product was purified and the radio-peptide activity was measured using a dose calibrator (Capintec) and expressed in mega-becquerels (MBq). Reverse phase high-performance liquid chromatography (RP-HPLC) with radiation detector (Shimadzu/Berthold) was used to determine the final product radiochemical purity using the method described in our previous study [9].
Cell lines
The Ga-68 labelled somatostatin analogue peptide was tested on two different cancerous cell lines: U87MG (glioblastoma) and AR42J (pancreatic adenocarcinoma). AR42J was cultivated in Ham’s F12 medium supplemented with 10% FBS, L-glutamine 2 mM, penicillin 100 U/ml, streptomycin
100 μg/ml. U87MG was cultivated in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS, L-glutamine 2 mM, penicillin 100 U/ml, streptomycin 100 μg/ml. For testing the affinity, Petri dishes were inoculated on one side of the dish with 400.000 cells of glioblastoma, respectively 500.000 cells pancreatic adenocarcinoma and left 24 hours before affinity experiments were carried out, in order for the cells to adhere to the plate surface.
Affinity and retention experiments
The affinity and retention of the synthesised peptide for the cultures somatostatin receptors were determined using a rotational radioimmunoassay method [10], using LigandTracer Yellow device, to determine the level of peptide bonded to cells, based on the increase in detected radioactivity. The Petri dish inoculated with cells was placed in the device on the rotating platform. The program used was set to measure the activity in 12 points, sets of 6 points in 2 opposite sides of the dish, one with cells and the one without.
For affinity testing the culture dish with fresh medium was rotated in order to acquire a background radiation reading without the radioligand. 100 μl of radiolabelled peptide solution (1,129 nmol of 68Ga-NODAGA-NOC) was added to the culture, the activity inoculate was approximately 15.5-18.5 MBq. The data acquisition phase of the program was carried out until a saturation of the peptide on the cells was observed on the resulting graph, at which point the data acquisition was paused. The medium was removed and the culture were washed twice with fresh medium to remove any free radioligand from the dish, 2 ml of fresh medium were added and the data acquisition was restarted in order to assess the retention time of the peptide bonded on the cells receptors.
Cell viability
The cell viability was tested using the MTT assay. Culture flask with 400.000 U87MG cells, respectively 500.000 AR42J cells, was prepared 24 hours before testing. The cells were incubated for
1 hour with the same activity of radioligand as in the affinity and retention tests. After the incubation, the medium was removed, the cultures were washed with fresh medium, and the medium with MTT reactive was added and incubated for 3 hours. The samples were analysed at 570 nm [11].
3. Results
Synthesis and quality control of the radioligand
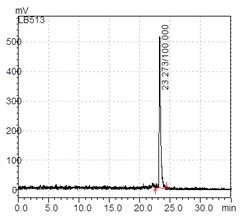
During the labelling process of peptides using high temperatures there is a risk of fragmentation, resulting in the formation of radioligands with different dimensions. The radio-chromatography is used to determine if the final product solution contains besides the expected peak of the radiolabelled peptide, free radioisotopes or fragments of labelled peptides. For medical application a radiopharmaceutical must have the radiochemical purity of at least 95%. In the radio-chromatograph the only peak appears at 23.273 min, indicating a purity of 100% labelled intact peptide, as shown in Figure 2, ideally for receptor affinity evaluation.
Affinity and retention experiments
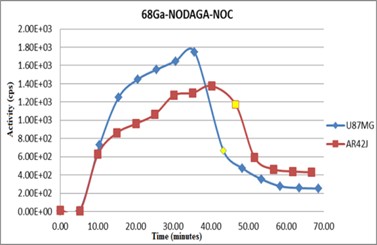
In the process of preparing the cell cultures for the in vitro tests, a lesser degree of adhesion on the plate surface was noted in the case of AR42J cells. In order to compensate and ensure a comparable number of cells with the U87MG cultures, the AR42J plates were inoculated with 500.000 cells instead of 400.000 cells. The LigandTracer Yellow graphs indicate the levels of the labelled peptides uptake (affinity test) on the cells receptors and the retention of the peptides, as shown in Figure 3.
The yellow point on the graphs represent the moment when the affinity testing ended and the analysis of peptide retention time was initiated. Comparing the two graphs we determined a higher concentration of peptide uptake on the U87MG cells, but also a lower retention of the peptide on the receptors. In the case of AR42J cells the peptide affinity was lower but the retention was far better, the first point of analysis for the retention after the medium with radioligand was removed presents a much lower drop compared with the level of peptide uptake.
Cell viability
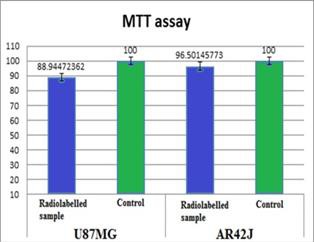
In order to assess if the radioligand used has any influence on the cells viability because of its ionising radiation, a cell viability test was performed, specifically the MTT assay. The MTT assay presented in Figure 4 indicates a small difference in cells viability between the two lines, in the case of pancreatic adenocarcinoma incubated with the radioligand the viability was 96.5% and in the case of glioblastoma was 88.94%. This indicates a slightly higher impact of 68Ga-NODAGA-NOC on cells viability on U87MG compared with AR42J.
4. Conclusions
68Ga-NODAGA-NOC is a peptide used for targeting of tumors with an overexpression of somatostatin receptor for medical applications. We determined that the radiolabelled peptide has a higher uptake on U87MG, but AR42J presents a better retention. Cell viability is slightly affected by the 68Ga-NODAGA-NOC, which is a first indicator of low radiotoxicity of the investigated agent for PET imaging, at investigational doses. Further test is to be performed for a full toxicological evaluation of 68Ga-labelled agents aimed for molecular imaging in cancer. The in vitro testing paves the path for the development and improvement of radiolabeled compounds in medicine for targeted diagnosis and therapy.
Acknowledgements
The work was supported through National Research Programme, UEFISCDI, contract number 68PCCDI/2018.
The Authors:
SERBAN Radu-Marian [1] [2]
TEMELIE Mihaela [1]
NICULAE Dana [1]
DRAGANESCU Doina [3]
DINISCHIOTU Anca [2]
[1] Horia Hulubei National Institute for R&D in Physics and Nuclear Engineering, Radiopharmaceuticals Research Centre (ROMANIA).
[2] University of Bucharest, Faculty of Biology (ROMANIA).
[3] University of Medicine and Pharmacy Carol Davila, Faculty of Pharmacy (ROMANIA).
Contributo selezionato da Filodiritto tra quelli pubblicati nei Proceedings “17th Romanian National Congress oh Pharmacy – 21st Century Pharmacy – Between Intelligent Specialization and Social Responsibility - 2018”
Per acquistare i Proceedings clicca qui.
Contribution selected by Filodiritto among those published in the Proceedings “17th Romanian National Congress oh Pharmacy – 21st Century Pharmacy – Between Intelligent Specialization and Social Responsibility - 2018”
To buy the Proceedings click here.



