Glaucoma medication decrease after Laser-Based treatment

Abstract
Purpose
Glaucoma can be controlled with local medication, laser therapy, surgery or a combination of these methods. Because of the fact that vision loss from glaucoma is irreversible, any treatment target is to prevent vision loss. Our study objective is to evaluate the amount of medication used after performing micropulse transscleral diode laser cyclophotocoagulation (mTSCPC) in patients diagnosed with glaucoma.
Material and Methods
This open-label nonrandomized non-comparative study, which included a number of 40 eyes of 40 patients, evaluates the amount of medication used after the laser treatment. The intraocular pressure (IOP) and medication used were monitored for 3 months.
Results
The mean ±SD baseline IOP was 36.50±13.38 mmHg, after 1-week IOP decreased significantly at 20.50±10.25 mmHg. During the successive postoperative visits (1 month, 3 months), the average IOPs remained stable compared to the preoperative (baseline) value. At the final monitored visit (3 months after the mTSCPC treatment) the measured IOP was 23.09±9.63 mmHg.
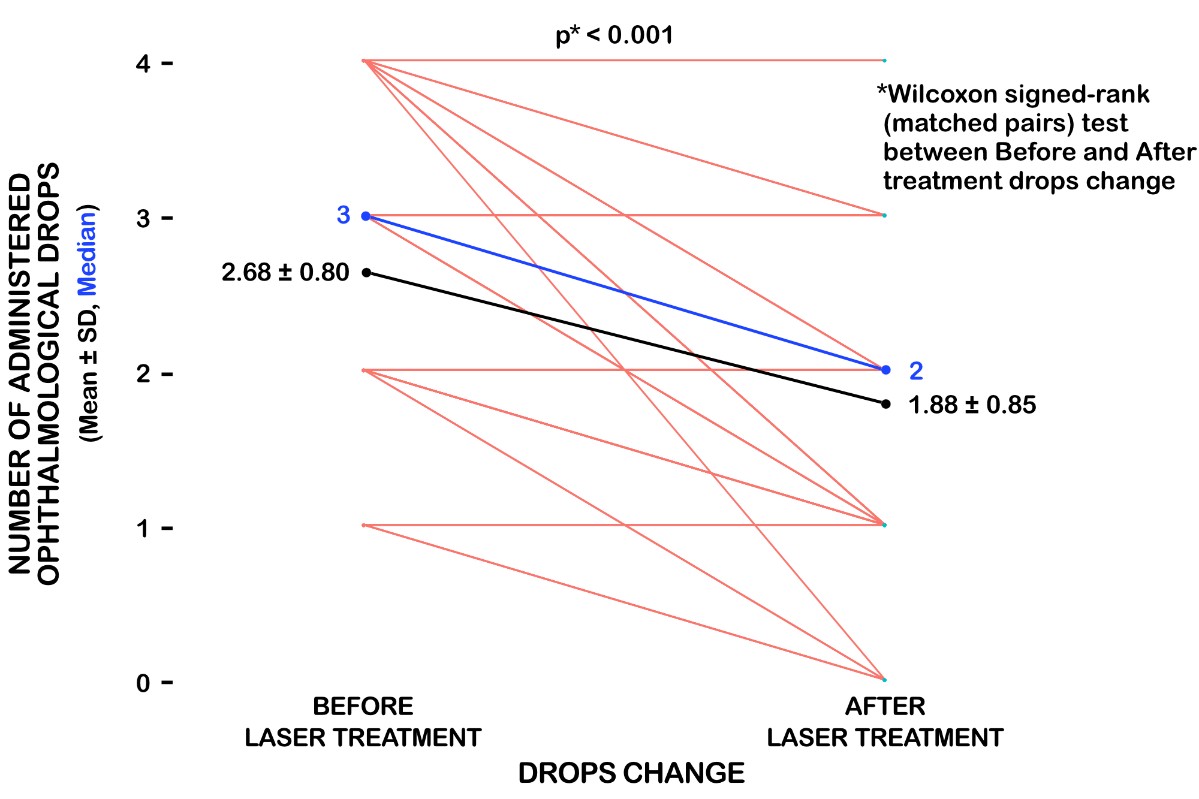
Mean medication value at baseline was 2.68±0.80, and at final visit, 3 months after the procedure, the mean medication value was 1.88±0.85.
Conclusions
Glaucoma medication can be reduced after performing micropulse transscleral cyclophotocoagulation with diode laser, offering a better compliance to treatment.
Table of Contents:
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusion
1. Introduction
There are many options available to treat glaucoma. These include local drops, laser procedures, and surgery. All are intended to decrease the intraocular pressure (IOP) and protect the optic nerve. Eye drops are usually the first-choice treatment [1]. Very often a combination of medications and laser treatment can control the IOP for a long time.
Types of glaucoma medication: prostaglandin analogs, beta blockers, alpha agonists, carbonic anhydrase inhibitors (CAIs), rho kinase inhibitors and combined medications.
For some patients who cannot tolerate medications or for whom medication alone is insufficient, laser treatment represents an excellent alternative [2]. Laser treatments for glaucoma are selective laser trabeculoplasty (SLT), argon laser trabeculoplasty (ALT), conventional laser cyclophotocoagulation and micropulse transscleral diode laser cyclophotocoagulation (mTSCPC).
Previous clinical and experimental studies have demonstrated that diode laser micropulsing has had good results in intraocular pressure reduction without tissue damage and diminishing collateral thermal tissue injuries [3-6]. Micropulse transscleral diode laser cyclophotocoagulation (mTSCPC) delivers the laser in repetitive short pulses of energy with “off, pause” periods in- between.
2. Material and Methods
Our study is an open label, non-randomized trial to evaluate the amount of medication needed to control the IOP after performing transscleral micropulse laser cyclophotocoagulation in patients diagnosed with glaucoma. 40 eyes of 40 patients were treated and monitored for a period of 3 months after the laser procedure, during 2018. All patients were under glaucoma pharmaceutical treatment, which included 3 types of eye drops, consisted of 4 classes of medication. Inclusion and exclusion criteria were established.
Inclusion criteria:
• Patients with glaucoma, with IOP>21 mmHg;
• Signed informed consent;
• Age 18-80 years. Exclusion criteria:
• Any ocular surgery or laser treatment in the past year;
• Any general diseases that might influence the ocular pressure.
The laser procedure was done in the operating theatre, under retrobulbar anaesthesia with 1:1 mixture of 0.75% bupivacaine and 4% lidocaine. Laser settings were 2000 mW of 810 nm infrared diode laser set on micropulse delivery mode, both hemispheres were treated for 80-130 s. The duty cycle was 31.3%, which means 0.5 ms of “on time” and 1.1 ms of “off time”. The protocol applied consisted of a sliding motion with the probe, from 10 o’clock to 2 o’clock for the superior hemisphere, and from 4 o’clock to 8 o’clock for the inferior hemisphere.
Ocular examinations were performed at 1 week, 1 month and 3 months after the treatment, and included anterior segment examination, Goldmann applanation tonometry and number of glaucoma medication. Only one laser application was done. Postoperatively, topical drops of prednisolone acetate 1%, tobramycin and cyclopentolate were prescribed for 2 weeks. At the 1- month visit, depending on the IOP glaucoma treatment was adjusted.
Statistical analysis and parameter comparisons were performed using Wilcoxon rank sum test (matched pairs).
3. Results
Fourty eyes of 40 patients were included in the study, during 2018, and underwent micropulse transscleral diode laser cyclophotocoagulation. The follow-up period was of 3 months. The mean ±SD baseline IOP was 36.50±13.38 mmHg, after 1-week IOP decreased significantly at 20.50±10.25 mmHg. During the successive postoperative visits (1 month, 3 months), the average IOPs remained stable compared to the preoperative (baseline) value. At the final monitored visit (3 months after the mTSCPC treatment) the measured IOP was 23.09±9.63 mmHg. Mean medication value at baseline was 2.68±0.80, and at final visit, 3 months after the procedure, the mean medication value was 1.88±0.85.
The amount of medication was at baseline 2.68±0.80, and at three months was 1.88 ± 0.85 (Fig.1). We started to reduce the medication with the prostaglandin analogues.
4. Discussion
Glaucomatous features include: progressive damage of the visual field, optic neuropathy and uncontrolled IOP. Several studies have shown an association between visual field loss and health related quality of life of glaucoma patients [7-9]. For some reasons, some patients refuse after a period of time, to administrate all the glaucoma treatment needed to control their IOP, and other treatment options are to be considered in order to preserve their visual acuity [10].
Often patients with glaucoma have associated pathology, and one of our inclusion criteria was to evaluate other diseases and might influence patients IOP control. General consultations for all enrolled patients were performed [11-23].
Micropulse diode laser transscleral cyclophotocoagulation gives the possibility of lowering IOP with minimal collateral damage and complications [24, 25]. In our study, the mean IOP drop was approximate 36%, and the medication was reduced by 29,9% in accordance with the literature studies [26, 27].
Regarding complications noted after the laser procedure, 10% of the patients had an inflammatory reaction of the anterior chamber for 2 weeks after the treatment. The existing studies conclude that the IOP reduction produced by the micropulse TSCPC is steady over time and medication can be successfully reduced [3, 6, 27].
5. Conclusion
Laser based procedure is a feasible procedure, that can safely be used for lowering IOP and to reduce the glaucoma medication, improving patient’s quality of life. All in all, our study the average IOP at the final visit was 23.09±9.63 mmHg mmHg and the mean medication was 1.88±0.85.
Our study demonstrates the possibility to reduce glaucoma medication and control IOP, even if it has its limitations, regarding the number of patients and the follow-up period.
The Authors:
ȘUȚĂ Marius Cristian [1]
KARANCSI Olimpiu Ladislau [2]
ARDELEAN Marius [1]
[1] PhD student, Department of Ophthalmology, “Victor Babeş” University of Medicine and Pharmacy, Timisoara, (ROMANIA).
[2] Department of Oral Implantology and Prosthetic Restorations on Implants, “Victor Babes” University of Medicine and Pharmacy, Timisoara, (ROMANIA).
Contributo selezionato da Filodiritto tra quelli pubblicati nei Proceedings “11th Conference of Ophthalmology with International Participation - 2019”
Per acquistare i Proceedings clicca qui.
Contribution selected by Filodiritto among those published in the Proceedings “11th Conference of Ophthalmology with International Participation - 2019”
To buy the Proceedings click here.



