Helicobacter pylori: old and new challenges in the microbiological diagnosis

Abstract
Background and Aim
Helicobacter pylori (HP) is an oncogenic agent, involved in preventable upper digestive malignant or premalignant conditions. The use of antibiotic therapy to eradicate HP infection, as indicated by guidelines, may be ineffective if resistance to them is unknown. The aim of this work was to cultivate HP from gastric biopsies and to perform antibiotic susceptibility testing.
Materials and Methods
Between June and December 2018, a sample of patients with suggestive gastric symptoms consulted at the 2nd Medical Clinic in Cluj-Napoca, Romania, who exhibited positive HP fecal antigen and agreed to undergo gastric endoscopy and prelevation of biopsy specimens for bacterial culture, have been included in this study. Biopsy specimens collected for bacterial culture were transported to the laboratory for cultivation. Special growth conditions have been ensured: microaerobic atmosphere, selective media, and longer growth time compared to other bacteria (10-14 days). For all isolated strains antibiotic susceptibility testing was performed using E-tests for clarithromycin, levofloxacin, metronidazole, amoxicillin and disc diffusion method for tetracycline and rifamycin.
Results
Twenty-six adult patients (7 men, 19 women) underwent gastric endoscopies and gastric biopsies during the recruitment period. After sample processing and culture, only 8 strains of HP were obtained, despite the fact that all investigated patients presented positive HP fecal antigen.
One strain was resistant to 3 antibiotics, two strains were resistant to 2 antibiotics, 4 strains to one antibiotic. Six of the eight isolated HP strains were resistant to metronidazole (75%), three were resistant to levofloxacin (37.5%), one was resistant to amoxicillin (12.5%) and one was resistant to clarithromycin (12.5%).
Conclusions
Isolation of HP after gastric biopsy allows for antibiotic susceptibility testing, however, successful cultivation of HP raises a number of difficulties. Given the small number of HP strains isolated so far in this study, the above figures regarding antibiotic resistance are a very rough and preliminary approximation that will be refined as our study continues.
Table of Contents:
1. Introduction
2. Materials and Methods
3. Results
4. Discussions
5. Conclusions
1. Introduction
Helicobacter pylori (HP) is a first-degree oncogenic agent [1], therefore once detected, it should be eradicated, in order to prevent the occurrence of neoplasia or precancerous conditions [2]. To eradicate HP infection, the most frequently used antibiotics (in association with gastric acid inhibitors) are macrolides, amoxicillin, metronidazole, tetracyclines, as well as bismuth. Some of these drugs can cause adverse effects, mainly gastrointestinal symptoms, causing poor patient adherence to treatment and anxiety. Therefore, antibiotics against HP should be used according to the best available evidence. On the other hand, the use of antibiotic therapy of HP as indicated by guidelines may be ineffective if resistance to them is unknown.
A main document has been published recently, the Maastricht V/Florence consensus [3], offering to both scientists and practitioners the current guidelines to cure HP infection. The emphasis is put on resistance to antibiotics, which can influence the type of prescription and the duration of therapy. The Maastricht consensus was preceded by the Kyoto consensus on HP gastritis [4], which established the necessity to cure HP infection in order to prevent conditions leading to gastric cancer and to decrease the incidence of gastric cancer.
Limited data is available on HP antimicrobial resistance in Eastern Europe [5]. Some data have been published in Bulgaria, Poland, and Russia, but a recent review showed several East European countries where relevant data are not available [6]. Romania has a prevalence of HP infection higher than 50% [7, 8, 9], therefore it is highly important to have information on the antibiotic resistance pattern of HP in Romania.
The irrational administration of antibiotics has led to the appearance of microbial resistance to antibiotics, which can be multiple and difficult to treat. It is necessary to know which HP strains present resistance to antibiotics, in order to prescribe the appropriate antimicrobial drugs. Given the present political and economic conditions, including population changes, traveling, mobility, immigration, there is an increasing and emergent need to determine the HP strains resistant to those antibiotics recommended by the Maastricht guidelines.
The use of antibiotic therapy to eradicate HP infection, as indicated by guidelines, may be ineffective if resistance to them is unknown. The aim of this work was to cultivate HP from gastric biopsies and to describe the antibiotic susceptibility pattern of isolated HP strains.
2. Materials and Methods
Between June and December 2018, a sample of patients with suggestive gastric symptoms consulted at the 2nd Medical Clinic in Cluj-Napoca, Romania, who exhibited positive HP fecal antigen (H. pylori antigen rapid test, Zhejiang orient Gene Biotech Co., LTD; Se=98.8%, Sp=100% relative to reference ELISA) and agreed to undergo gastric endoscopy and prelevation of biopsy specimens for bacterial culture, after informed consent, have been included in this study.
The study was conducted according to the 2013 revision of the Helsinki declaration (Fortaleza) and was approved by the research ethics committee of the Iuliu Hatieganu University of Medicine and Pharmacy (Nr. 163/2.04.2018).
Biopsy specimens (antrum and body of the stomach) collected for bacterial culture were transported in Portagerm pylori (bioMérieux) to the laboratory for inoculation onto commercial selective medium Pylori Agar (bioMérieux). Special growth conditions have been ensured: microaerobic atmosphere using gas generating sachets CampyGen™Compact system (Oxoid), 37 °C and longer growth time compared to other bacteria (10 days). Once incubated, the colonies resembling HP were identified by oxidase, catalase, urease tests and microscopy [10, 11].
For all isolated strains antibiotic susceptibility testing was performed using E-tests (bioMérieux) for clarithromycin (CH), levofloxacin (LEV), metronidazole (MZ), amoxicillin (AMX) and disc diffusion method for tetracycline (TET) and rifamycin (RIF).
For antibiotic susceptibility testing (AST), fastidious Mueller Hinton agar medium (MH-F) supplemented with 5% defibrinated horse blood and 20 mg beta-NAD (bioMérieux) was used [12], the inoculum was prepared from a 2- or 3-day old subculture grown on a blood agar plate and was adjusted to an opacity equivalent to 3 McFarland, approx. 108 colony forming units (CFU)/mL. The AST results were read after 48-72 h of incubation at 37 °C in microaerobic atmosphere, provided that growth was clearly visible. The MIC values of the antibiotics tested by E-tests (amoxicillin, clarithromycin, levofloxacin, metronidazole) were read where the inhibition ellipse intersects the strip. Interpretive criteria for Susceptibility vs. Resistance after E-tests were the following: clarithromycin ≤0.5 vs. >0.5 µg/mL, levofloxacin ≤1 vs. >1 µg/mL, metronidazole ≤8 vs. >8 µg/mL, amoxicillin <0.125 vs. >0.125 µg/mL [10, 12]. For the disc diffusion method, the interpretative criteria for Susceptibility vs. Resistance were: for tetracycline <17 mm vs. ≥19 mm and for rifamycin <14 mm vs. ≥19 mm [10]. The HP strain CCUG 17874 was used for quality control.
Data has been collected and described using Microsoft Excel 2010. Confidence intervals for proportions have been computed with continuity correction, using VassarStats [13].
3. Results
Twenty-six patients (7 men, 19 women) underwent gastric endoscopies and gastric biopsies during the recruitment period. After sample processing and culture, only 8 strains of HP were obtained, despite the fact that all 26 investigated patients presented positive HP fecal antigen. All clinical isolates were obtained from adult patients (one male and 7 women) aged between 33 and 66 years old.
Five HP strains were isolated from patients previously exposed to H. pylori eradication regimens. One HP strain, isolated from the youngest female patient (33 years old), who received previous eradication treatment, was resistant to 3 antibiotics (CH, LEV, MZ), two strains (one from a patient with previous eradication treatment) were resistant to 2 antibiotics (LEV and MZ), 4 strains (two from patients previously exposed to eradication treatment) were resistant to one antibiotic (3 HP strains to MZ and one strain to AMX). The main results obtained in this preliminary study are presented in Table 1.
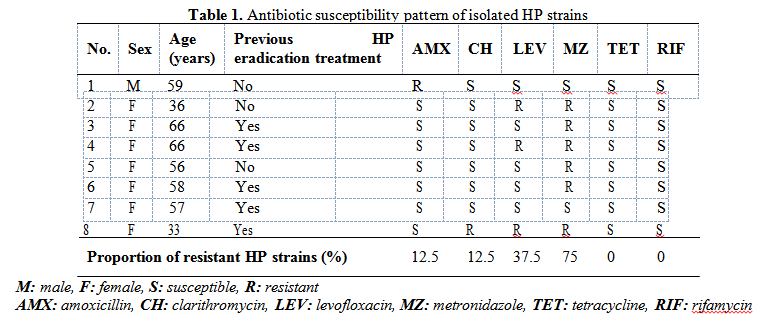
Six of the eight isolated HP strains (two from patients previously exposed to eradication treatment) were resistant to MZ (75%, 95% CI: 35.6-95.6%), three (two isolated form patients previously exposed to eradication treatment) were resistant to LEV (37.5% 95% CI: 10.2-74.1%), one (no previous treatment) was resistant to AMX (12.5%) and one (previously exposed to treatment) was resistant to CH (12.5% 95% CI: 0.7-53.3%). One HP strain isolated from a female patient with gastric cancer was susceptible to all tested antibiotics. All 8 HP isolated strains were susceptible to TET and RIF.
4. Discussions
Infection with H. pylori is present worldwide, more prevalent in developing (50.8%, 95% CI:
46.8-54.7%) compared to developed (34.7%, 95% CI: 30.2-39.3%) countries. In Europe, the highest prevalence rate was reported in Serbia (88.3%, 95% CI: 84.6-92.0%), whereas in Belgium (11.0%, 95% CI: 8.3-13.7%), and in Sweden (15.0%, 95% CI: 11.1-18.9%) the lowest infection rates were reported [14].
In Romania, the prevalence of H. pylori infection was reported to be higher than 50% [7, 8, 9], but no information has been published on HP antibiotic resistance pattern during recent years.
Romanian medical doctors are aware of the importance of HP infection and well trained to fight it [8].
Given this need to fight HP infection and given the recommendations of the Maastricht V/Florence to choose the antibiotics and the length of therapy according to the antimicrobial sensitivity, we need to know what strains respond to which drugs.
This work presents the preliminary results of a study that will try to fill some of the blank areas regarding HP resistant strains in Europe by cultivating HP from gastric biopsies and performing antibiotic susceptibility testing in patients from Cluj-Napoca, Romania.
For all 26 patients included so far, fecal HP antigen was positive, but only 8 strains of HP were isolated. Cultivation of HP can be fastidious, time consuming (up to two weeks until an antibiogram is obtained), and expensive. While culture sensitivity may reach 95%, the success of HP cultivation from gastric biopsies depends on multiples factors: whether the patients received treatment with antibiotics or antisecretory drugs, especially proton pump inhibitors (PPI) before the biopsy was taken, the number of biopsies, proper transportation and processing of biopsy specimens, selective media used for growth, the atmosphere and temperature during incubation [15]. It has been reported that culture has a lower sensitivity compared to real-time PCR from gastric biopsies [10]. Nevertheless, culture from gastric biopsies has a very high specificity, while phenotyping and genotyping can also be performed afterwards. Obtaining a pure HP culture from gastric biopsies will also allow antibiotic susceptibility testing against several antibiotics used in eradication treatment.
In this study, six of the eight isolated HP strains (two from patients previously exposed to eradication treatment) were resistant to MZ (75%). Other studies reported a high resistance to metronidazole: 45.9% resistance in France [10], 42% in Poland [16], 38.6% primary resistance in Italy [17], 24% primary resistance in Bulgaria [18]. Metronidazole is an effective antibiotic against infections produced by protozoa (e.g., Trichomonas vaginalis, Giardia), anaerobic bacteria (e.g., Clostridium difficile), so its use in the treatment of gastrointestinal, gynaecological, dental and parasitic infections can contribute to the increase in HP resistance [19]. Metronidazole resistance is the most prevalent pattern of HP resistance worldwide [20]. However, for MZ, E-tests reveal significantly higher minimum inhibitory concentration values compared to the dilution method [21].
Studies performed in several European countries reported HP primary and secondary resistance to clarithromycin. Resistance percentages varied between countries, all the Northern European countries had a resistance rate of <10% while all those from the rest of Europe except Germany and Spain had a resistance rate of >20% [5, 10, 16, 20]. So far, we only detected one (12.5%) HP strain resistant to CH, isolated from a patient exposed to eradication treatment.
In our work, three HP strains, two isolated form patients previously exposed to eradication treatment, were resistant to LEV (37.5%). Primary and secondary resistance to levofloxacin was reported in several studies: 30% in Turkey, 29% in Belgium, 18% in Germany, 16% in Spain, 15% in France [10, 20], 28.7% primary resistance in Italy, increasing from only 10.6% in 2007 [17, 22], and 8% in Lower Silesia, Poland [16].
The prevalence of HP resistance to AMX appears to remain low, less than 5% in most of the European countries [20], e.g., 0.7% in France [10], 1.1% primary resistance in Bulgaria [18]. We detected one (12.5%) HP strain resistant to amoxicillin, that has been isolated form a naïve patient.
All 8 isolated HP strains were susceptible to TET and RIF, consistent with other findings [10,16, 20].
One (12.5%) HP strain isolated from the youngest female patient (33 years old), who received previous eradication treatment, was resistant to 3 antibiotics (CH, LEV, MZ). Two (25%) HP strains (one strain from a patient with previous eradication treatment) were resistant to 2 antibiotics (LEV and MZ). Combined resistance to two or even three antibiotics has been previously described. In a study performed in Poland, the multidrug resistant strains (for two or more antibiotics) were often isolated from the youngest patients or from patients above 65 years of age: 20% strains were resistant to CH and MZ, 4% resistant to MZ and LEV, and 2% of strains were resistant to 3 antibiotics: CH, MZ and LEV [16]. Primary LEV+MZ resistance was 4.9% in an Italian study [18]. In a systematic review and meta-analysis regarding the prevalence of antibiotic resistance found in HP, combined resistance to CH and MZ reached 18% (95% CI, 16%-20%) in Europe [20].
Increased use of antibiotics in empirical treatment for microbial infections, HP included, can be correlated with an increase in microbial resistance [19, 23]. In order to improve the success rate of anti HP treatment it is important to determine the susceptibility pattern of isolated strains.
Molecular tools were developed for rapid detection of HP strains resistant to antibiotics, but these techniques are not available everywhere and few laboratories can determine routinely the molecular HP resistance pattern to all antibiotics that could be used for treatment [10, 15, 24].
Therefore, HP culture remains a valuable tool for HP identification and antibiotic susceptibility testing.
The main limitation of this preliminary study is represented by the small number of HP strains that have been isolated and tested so far. Nevertheless, as we continue recruiting and testing volunteers for this study, we will report our progress in future works.
5. Conclusions
Isolation of HP after gastric biopsy allows for antibiotic susceptibility testing, however, successful cultivation of HP raises a number of difficulties. Given the small number of HP strains isolated so far in this study, the above figures regarding antibiotic resistance in Cluj-Napoca, Romania are a very rough and preliminary approximation that will be refined as our study continues.
Acknowledgments
The authors wish to thank Prof. Dr. Francis Mégraud for his valuable guidance and support in planning and conducting this research.
Funding sources
Culturing and testing expenses were partially funded by the Romanian Society of
Neurogastroenterology.
E-tests were provided by bioMérieux, France. The HP quality control strain CCUG 17874 was provided by Prof. Dr. Francis Mégraud, Centre National de Référence des Campylobacters et Helicobacters, Laboratoire de Bactériologie, Université de Bordeaux, France.
The Authors:
COLOSI Ioana Alina [1]
COSTACHE Carmen [1]
GRAD Simona [2]
GRAD Cosmin [2]
LUCA Diana Maria [3]
COLOSI Horaţiu Alexandru [4]
DUMITRAŞCU Dan Lucian [2]
[1] Department of Molecular Sciences, Division of Microbiology, Iuliu Hațieganu University of Medicine and Pharmacy, Cluj- Napoca, (ROMANIA).
[2] Department of Internal Medicine, 2nd Medical Clinic, Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, (ROMANIA).
[3] Unirea Medical Center, Cluj-Napoca, (ROMANIA).
[4] Department of Medical Education, Division of Medical Informatics and Biostatistics, Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, (ROMANIA).
Contributo selezionato da Filodiritto tra quelli pubblicati nei Proceedings “Central European Gastroenterology Meeting (CEURGEM) - 2019”
Per acquistare i Proceedings clicca qui.
Contribution selected by Filodiritto among those published in the Proceedings “Central European Gastroenterology Meeting (CEURGEM) - 2019”
To buy the Proceedings click here.



