Minor Salivary Gland Carcinoma

Abstract
Introduction
Minor salivary gland tumors are not a frequent, accounting for only 10-15% of all salivary gland neoplasms. Most develop in the oral cavity, of which 50% are malignant. Wide surgical excision is the gold standard in the treatment of minor salivary gland tumors, with the relapse rate being increased in cases of incomplete exacerbation.
Materials and methods
The aim of this article is to present the surgical treatment and the modalities of reconstruction of the surgical wound after a large excision of a minor salivary gland carcinoma of a pacient from the “Prof. Dr. Dorin Hociota” Institute of Phlaooaudiology and Functional Surgery, Bucharest.
Results
The treatment of the pacient’s minor salivary gland carcinoma was complete surgical resection Due to the large tumor excision, it is decided to close the defect with autologous flaps: the flap of the right pectoralis major muscle and the sternocleidomastoidian muscles with the tegumentary flap from the right supraclavicular fossa.
Conclusions
Tumors of the minor salivary glands, although they are not a common pathology, representing only 10-15% of the total salivary gland neoplasms and have an aggressive progression, half of the neoplasms being malignant. The standard treatment of minor salivary gland tumors is complete surgical excision, because the relapse rate is increased. Surgical defects following extensive resections can be repaired through musculo-cutaneous pedicled flaps. Depending on the location and size of the defect, the most used flaps are pectoral (pectoralis major muscles), deltopectoral flap or sternocleidomastoidian muscle.
Table of Contents:
1. Introduction
2. Materials and methods
3. Results
4. Discussions
5. Conclusions
1. Introduction
Salivary gland tumors occupy 3-5% of all head and neck neoplasms [1]. Minor salivary gland tumors represent 10-15% of all salivary gland neoplasms. These can occur in the palate, paranasal sinuses and nasal cavity, tongue, floor of the oral cavity, gums, pharynx, larynx or trachea. More than half of the tumors are located at the level of the oral cavity [2].
Tumors of minor salivary glands represent a heterogeneous group of tumors with a wide histopathological diversity [1]. Currently, the WHO classification of salivary gland tumors, published in 2005, is widely accepted and used by anatomopathologists worldwide [3].
Half of the neoplasms of the minor salivary glands are malignant. Their most frequent locations are the mouth and the retromolar trigone, followed by the tongue and the lower lip [1].
Clinical manifestations depend on the location and size of the tumor. Frequently, minor salivary gland tumors appear like lumps. Clinical signs suggestive of malignancy include pain, elevated growth rate (<1 year), adherence to deep and/or superficial planes, superficial skin changes, ulceration, bleeding, and enlarged lymph nodes [1].
Paraclinic investigations recommended for the diagnosis of salivary tumors are CT or MRI, the last one having the advantage of removing dental artifacts. The diagnosis of certainty is on the histopathological examination of the intraoperative biopsy or the fine needle aspiration biopsy – FNAB [1].
Staging TNM of a salivary tumor is very important in establishing the patient’s therapeutic attitude and prognosis [4]. According to NCCN (National Comprehensive Cancer Network) guide, the standard treatment of salivary neoplasms is surgical tumor excision. Inoperable tumors, patients who refuse surgery or are not eligible for general anesthesia due to comorbidities, are advised to initiate radiotherapy. Chemotherapy is indicated in palliative, unresectable relapsed tumors, non-radiotherapy and metastatic patients [2].
Tumors of minor salivary glands have an increased relapse rate in case of incomplete surgical excision (5-30%). Therefore, extensive tumor resections and postoperative follow-up are recommended to prevent recurrences [1].
2. Materials and methods
A 62-year-old patient presented at the Institute of Phonoaudiology and Functional ENT Surgery “Prof. Dr. Dorin Hociota” for a painful, ulcerated, infected right cheek swelling with an onset of about 6 months (Fig. 1). From the personal pathological history of the patient we retain type II diabetes mellitus insulin-dependent and high blood pressure in treatment.
The clinical examination describes the infiltrative and vegetative tumor in the retromolar trigon extended to the right jugal mucosa and the right palatal tonsil, exteriorized to the right cheek, ulcerated and painful, with a diameter of about 3/4 cm.
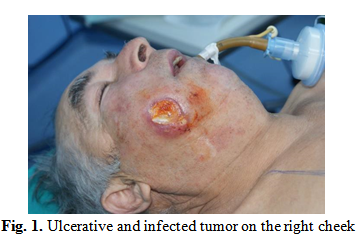
A cranio-cervical MRI was performed which allowed for a tumor staging of T4a N0 M0. The wide and complete resection of tumor was decided. A systemic antibiotic therapy with
large spectrum, anti-inflammatory and antialgic therapy was established.
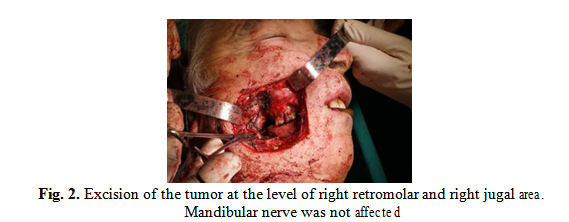
The preanesthetic consultation with operator risk assessment associated with patient comorbidities and patient informed consent were obtained prior to surgery. The intervention was performed under general anesthesia with oro-tracheal intubation and consisted of excision of the right retromolar and right jugal tumor and right adenoidectomy (Fig. 2). The resected surgical piece was sent for histopathological examination.
Due to the large tumor excision area we decided to close the defect with autologous flaps: the flap of the right pectoralis major muscle (Fig. 3) and the sternocleidomastoidian muscle with the tegumentary flap from the right supraclavicular fossa.
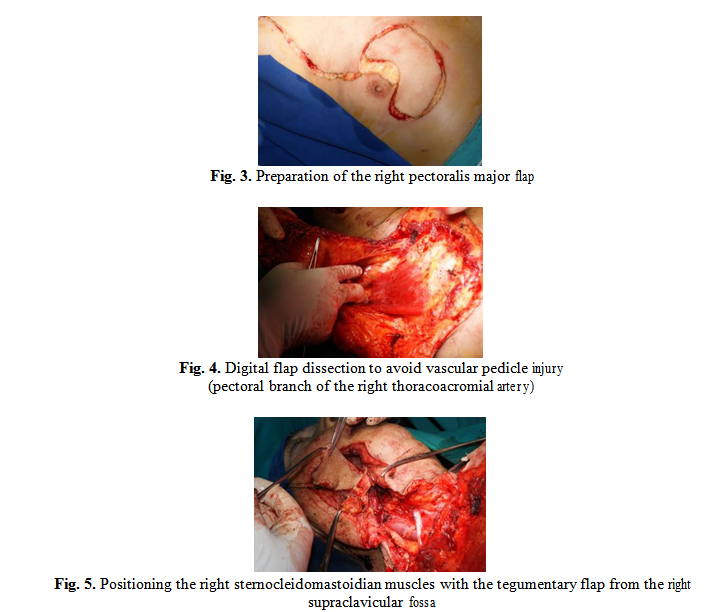
3. Results
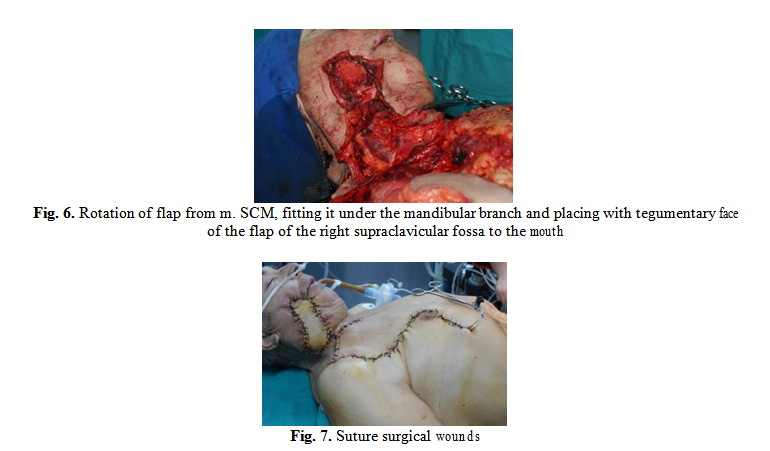
The patient received both solids and fluids via a nasogastric tube for 14 days. Strict glycemic control and daily care of surgical wounds and oral cavity were performed. A favorable postoperative progression was obtained.
The result of the histopathological examination was invasive squamous cell epidermoid carcinoma.
4. Discussions
Pediculous musculo-tegumentary flaps are important ways of reconstructing defects resulting from extensive surgical resections.
The pectoralis major myocutaneous flap (PMMF) is one of the most used techniques for reconstruction after head and neck surgery, being easy and fast to harvest. It has the advantage of being in the proximity of the head and neck, allowing the reconstruction after extensive excisions in oncological surgery.
Among the disadvantages of PMMF is the reduction in neck mobility and the need to rotate it to 180° when using a skin paddle, which can affect the flap’s vascular supply. Also, the excess subcutaneous fat between the pectoral muscles and the skin may limit the phonation and swallowing [13].
The postoperative complications of the flap are: flap necrosis, fistula, wound dehiscence, hematoma and infection. These complications may be favored by associated systemic diseases (e.g. diabetes), large resections or radiotherapy [14].
5. Conclusions
Tumors of the minor salivary glands, although not a common pathology, representing only 10-15% of the total salivary gland neoplasms, can have an aggressive progression, half of the neoplasms being malignant. The standard treatment of minor salivary gland tumors is complete surgical excision, because the relapse rate is increased. Surgical defects following extensive resections can be repaired through musculo-cutaneous pedicled flaps. Depending on the location and size of the defect, the most used flaps are pectoral (pectoralis major muscles), deltopectoral flap or sternocleidomastoidian muscle.
Authors:
Viorel Zainea
Andreea Rusescu
Cătălina Pietroșanu
Dragoș Cristian Ștefănescu
Silviu Pițuru
Irina Gabriela Ioniță
Oana Ruxandra Iana
Mahmoud Daoud
Răzvan Hainăroșie
Flavia Alexandra Iacobescu



